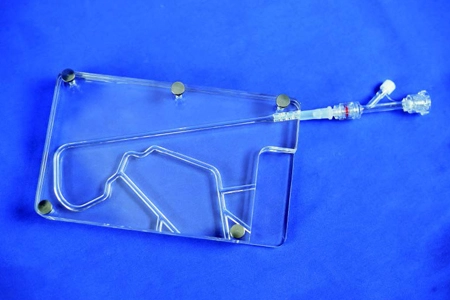
China'S First Cardiovascular Implant Device Usability Testing System
China's first cardiovascular implant device usability testing system completed delivery to Jiangsu Medical Device Inspection Institute (Suzhou)
--Helping to improve the usability testing capability of cardiovascular implantable devices in China.
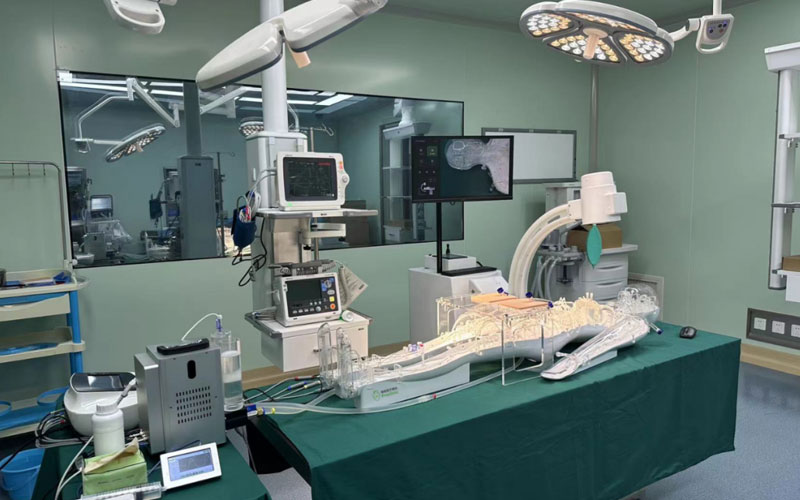
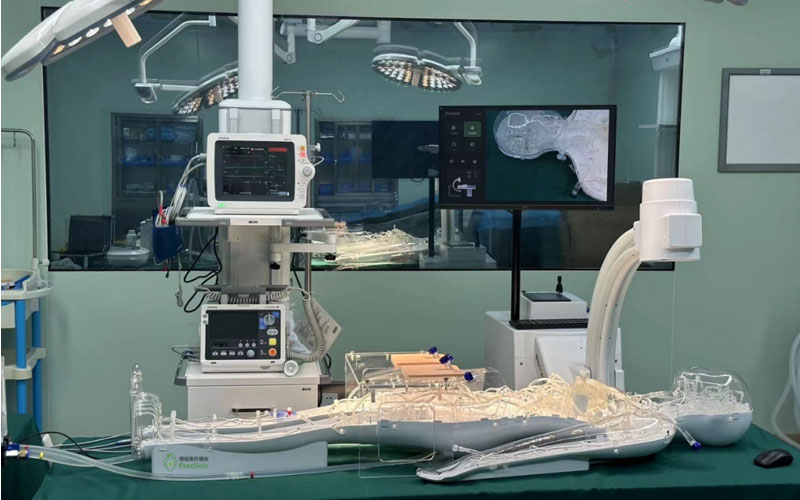
On 25 May 2023, the usability testing system for cardiovascular interventional devices at the Usability Testing Research Centre of the Suzhou Branch of the Jiangsu Medical Devices Laboratory was installed and put into trial, marking a new step in the usability testing of cardiovascular implant interventional devices in China. As the first usability testing platform for medical devices in China, the usability testing platform for medical devices in Jiangsu Province will provide strong support for the usability research and testing of cardiovascular implant devices in China.
The usability testing system for cardiovascular interventional devices was developed by the Jiangsu Medical Device Usability Testing Research Centre and designed and manufactured by Shanghai Preclinic Medtech, which can meet the human-machine interaction, ergonomics, ergonomics, usability testing and user experience of cardiovascular implant devices. The innovative testing system has been declared as an invention patent.
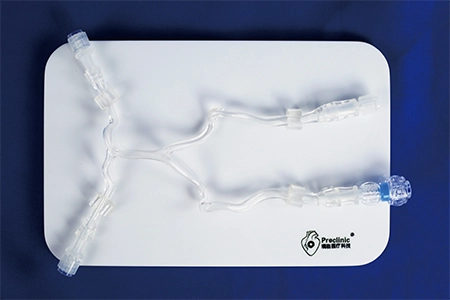
About usability testing of medical devices
Medical device usability testing is the use of a device by a representative user to perform a representative task to reveal the interaction benefits and opportunities for improvement. The aim is to understand whether the device meets the user's needs and, crucially, the need for safe operation, through stress testing or troubleshooting of the device's user interface. Design improvements and design validation are achieved through design changes to confirm that they do not cause hazardous use errors. Usability testing identifies opportunities for improvement to make the operation of medical devices easier, safer, more effective and more usable. This improvement in the quality of human-machine interaction will benefit everyone involved with the given device. The most profound reason for conducting usability testing of medical devices is to prevent people from being injured or killed as a result of misuse. The results of usability testing are used as a basis for design and development changes to the device, and records are kept.

About the Jiangsu Medical Device Usability Testing Platform
The Jiangsu Medical Device Usability Testing Platform is the first sharing platform jointly built by the government, enterprises, universities and international certification bodies in China. It is dedicated to improving the design level of medical devices and reducing the risk of medical device use in China, and plays an important role in improving the level of scientific and technological support for regulatory departments and helping the high-quality development of the medical device industry in Jiangsu and the whole country. It is a fruitful and positive attempt to integrate domestic and international innovation resources and explore the construction of a sharing platform.
At present, the platform has become the first professional full simulation medical environment usability testing platform in China, which can carry out a number of tests involving interaction design, human-machine interaction, human-machine interface, human factors engineering, ergonomics, usability testing and user experience, realizing a global leading laboratory with professional standards of user-centered and medical device usability testing.

About Shanghai Preclinic Medtech
Shanghai Preclinic Medtech was officially established in 2017, located in Qingpu Industrial Park, Shanghai, is a high-tech enterprise formed by a team of senior medical experts and engineers. We are committed to providing customized and standardized simulation medical products for medical professionals in the development and testing of surgical instruments and procedures, solution demonstration, and skills training, to accelerate technological innovation and implementation, and to help promote surgical technology.
Since 2014, the company's core team, led by Professor Wang Chengtao from the School of Mechanics and Dynamics of Shanghai Jiao Tong University, has been providing 3D printing-based medical-industrial integration technology services to a number of hospital clinical departments, and has completed many pioneering projects in the industry. The company currently has a team of professional clinical consultants, senior product design, manufacturing and software development teams, providing professional medical simulation products for minimally invasive interventional departments such as cardiovascular intervention, respiratory, digestive and urological departments. Our products have served more than 300 medical industry customers at home and abroad, with more than 2/3 of our customers being long-term customers, and we have also established long-term relationships with more than 50 hospitals, medical schools, medical device R&D and testing institutes.
Popular Medical Simulators
Related Medical Simulator Article

 English
English  中文
中文